We have had quite a journey in these 24 years! Sit back and travel through our major milestones.

Incorporation of our Company.
Our R&D arm, Bigtec Private Limited, one of our Subsidiaries commenced its operations.
Entered into a joint venture agreement dated August 1, 2011 with Bigtec Innovations Private Limited and Bigtec Private Limited.

In 2015, Molbio Diagnostics and Bigtec Labs merged, marking a new era in MedTech innovation. This strategic move transformed Bigtec into Molbio’s dedicated R&D wing, strengthening its diagnostics and technological development capabilities. With Bigtec driving innovation and Molbio ensuring rapid market adoption, they reinforce their shared vision of revolutionizing global healthcare.

In 2015, the H1N1 outbreak affected nearly 30,000 people in India. As a multi-disease real-time PCR, Truenat demonstrated its capabilities as a technology solution that can adapt itself to disease outbreaks within no time. With rapid and precise testing at the point of care, it played a crucial role in early diagnosis and containment, effectively cutting down the chain of transmission and becoming a silent warrior in the fight against the epidemic.

In 2016, India reeled under a massive outbreak of Chikungunya, with 64,057 confirmed cases across the country. Truenat, as a multi-disease platform, was utilized to deliver rapid results ensuring early detection and infection control, proving itself as an innovation that is adaptable and future-ready.

Launch of fully- automatic PCR device ‘Truelab Uno” and approved by the Indian Council for Medical Research (“ICMR”).

Received ‘License to manufacture for sale and distribution of medical device’ for chip based real time PCR test for hepatitis B virus, hepatitis A virus, hepatitis C virus and hepatitis E virus from the Central Drugs Standard Control Organisation, Ministry of Health, Government of India.
Received ‘License to manufacture for sale and distribution of medical device’ for chip based real time duplex PCR test for human papillomavirus high risk types 16, 31 and 18, 45 from the Central Drugs Standard Control Organisation, Ministry of Health, Government of India.
Investment by India Business Excellence Fund III in the Company.

Validation of the indigenous Truenat assay for tuberculosis by the NTEP.
World Health Organisation endorsed Truenat as the primary diagnostic test for MTB and MTB-RIF.
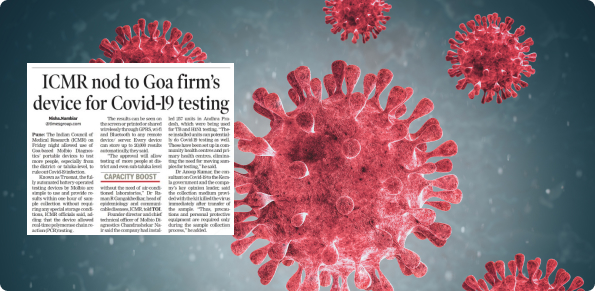
Received ‘License to manufacture for sale and distribution of medical device’ for chip based real time PCR test for beta coronavirus from the Central Drugs Standard Control Organisation, Ministry of Health, Government of India.
Received ‘License to manufacture for sale and distribution of medical device’ for chip based real time PCR test for HIV-1 virus from the Central Drugs Standard Control Organisation, Ministry of Health, Government of India.
Investment by V Sciences Investments Pte. Ltd in the Company.

Acquisition of Prognosys Medical Systems Private Limited to venture into radiology equipment.
Acquisition of Prognosys Healthcare (India) Private Limited.


Truenat became part of innovations rolled out under the Introducing New Tools Project (iNTP), a collaboration between USAID, and the Stop TB Partnership across 11 TB high-burden countries. Molbio also began collaborating with the Global Fund, a multilateral funding agency for global health that pools the world’s resources to invest strategically in programs to fight major killers in low—and middle-income countries: HIV, tuberculosis (TB), and malaria. To date, Molbio holds long-term agreements with both the United Nations and the Global Fund allowing countries to quickly access its products.
Launched ‘Edge’, a scaleup partnership initiative between our Company and Bigtec
Investment of 19.68% of the paid-up equity share capital of Optrascan, INC to diversify to digital pathology scanners

Molbio recognizes the importance of a level playing field for innovations and introduced the EDGE Program- a program designed to bridge the critical gaps between innovation and market adoption for point-of-need diagnostic technologies by integrating experience, expertise, and state-of-the-art facilities. The program aims to foster collaboration with researchers, healthcare professionals, and industry leaders to accelerate groundbreaking technology solutions.
Connect with us
Molbio Diagnostics Limited
(Formerly Molbio Diagnostics Private Limited)
© All Rights Reserved
PLEASE READ THIS NOTICE CAREFULLY. IT APPLIES TO ALL PERSONS WHO VIEW THIS WEBSITE. THESE MATERIALS ARE NOT DIRECTED AT OR INTENDED TO BE ACCESSED BY PERSONS LOCATED OUTSIDE INDIA. THESE MATERIALS ARE BEING MADE AVAILABLE ON THIS WEBSITE TO COMPLY WITH SECURITIES AND EXCHANGE BOARD OF INDIA (ISSUE OF CAPITAL AND DISCLOSURE REQUIREMENTS) REGULATIONS, 2018, AS AMENDED.
Investors should read the Draft Red Herring Prospectus and seek professional advice before taking any action.
IMPORTANT: You must read and agree with the terms and conditions of the following disclaimer before continuing.
The following disclaimer applies to the draft red herring prospectus of Molbio Diagnostics Limited (the “Company”) dated August 22, 2025 (the “Draft Red Herring Prospectus”) filed with the Securities and Exchange Board of India (“SEBI”) and hosted on this website in connection with the initial public offering of the equity shares (“Equity Shares”) of the Company (the “Offer”). You are advised to read the following notice carefully before accessing the Draft Red Herring Prospectus. In accessing the Draft Red Herring Prospectus, you agree to be bound by the following terms and conditions, including any modifications to them from time to time.
Access to the Draft Red Herring Prospectus does not constitute a recommendation by the Company, the members of the Syndicate (as defined in the Draft Red Herring Prospectus) or any of their respective affiliates or any other person to subscribe to the Equity Shares offered in the Offer.
The Draft Red Herring Prospectus is directed at, and is intended for distribution to, and use by, residents of India only. The information in this portion of our website, including the Draft Red Herring Prospectus, is not for publication or distribution, directly or indirectly, in or into the United States. The contents of the Draft Red Herring Prospectus is for your information only, and you acknowledge that access to the Draft Red Herring Prospectus is intended for use by you only and you agree not to forward the Draft Red Herring Prospectus on to any other person, internal or external to your company, in whole or in part, or otherwise provide access via e-mail or otherwise to any person. Any person into whose possession the DRHP comes is required to inform himself or herself about and to observe any such restrictions. BRLMs and/or its affiliates or their respective directors, officers and employees are not soliciting any action based on any of the information contained on this website, including the DRHP, and such information should not be construed as an offer, or invitation or offer to sell or the solicitation of any offer to buy or subscribe for or purchase any security. No part of the contents of the Draft Red Herring Prospectus shall be copied or duplicated in any form by any means or redistributed.
The Draft Red Herring Prospectus has been hosted on this website as prescribed under Regulation 26(1) of the Securities and Exchange Board of India (Issue of Capital and Disclosure Requirements) Regulations, 2018, as amended. Our Company has taken all necessary steps to ensure that the contents of the Draft Red Herring Prospectus as appearing on this website are identical to the Draft Red Herring Prospectus filed with the SEBI. You are reminded that documents transmitted in electronic form may be altered or changed during the process of transmission and consequently, neither the Company nor the BRLMs any of its affiliates accepts any liability or responsibility whatsoever in respect of alterations or changes which have taken place during the course of transmission of electronic data.
The Draft Red Herring Prospectus does not constitute an offer to sell or an invitation to subscribe to the securities offered in any jurisdiction to any person to whom it is unlawful to make an offer or invitation in such jurisdiction and is not intended for distribution to, or use by, any person or entity in any jurisdiction or country where (a) distribution or use of such information would be contrary to law or regulation; or (b) the Company or any of its affiliates would by virtue of such distribution become subject to new or additional registration, licensing or other regulatory requirements. The Equity Shares offered in the Offer have not been and will not be registered under the U.S. Securities Act of 1933, as amended (“U.S. Securities Act”) or any state securities laws in the United States, and unless so registered, may not be offered or sold within the United States, except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the U.S. Securities Act and in accordance with any applicable U.S. state securities laws. Accordingly, the Equity Shares are being offered and sold only outside the United States in ‘offshore transactions’ in compliance with Regulation S under the U.S. Securities Act and the applicable laws of the jurisdictions where such offers and sales are made.
Any person into whose possession the Draft Red Herring Prospectus comes is required to inform himself or herself about and to observe any such restrictions. Neither the Company, BRLMs nor any of its affiliates is soliciting any action based on the Draft Red Herring Prospectus, and it should not be construed as an offer to sell or the solicitation of an offer to buy any securities. Potential investors should not rely on the Draft Red Herring Prospectus for any investment decision.
Any decision on whether to invest in the equity shares described in the Draft Red Herring Prospectus may only be made after a red herring prospectus has been filed with the Registrar of Companies, Goa, Daman and Diu at Panaji and the SEBI and must be made solely on the basis of such red herring prospectus, as there may be material changes in the red herring prospectus compared to the Draft Red Herring Prospectus. Invitations to subscribe to or purchase the equity shares in the Offer will be made only pursuant to the red herring prospectus if the recipient is in India or the preliminary offering memorandum for the Offer, which comprises the red herring prospectus and the preliminary international wrap for the Offer, if the recipient is outside India. No person outside India is eligible to Bid for equity shares in the Offer unless that person has received the preliminary offering memorandum for the Offer, which shall contain the selling restrictions for the Offer outside India.
Any potential investor should note that investment in equity shares involves a high degree of risk and for details relating to such risk, please see the section titled “Risk Factors” of the red herring prospectus, when available.
Neither the Company, BRLMs, nor any of its affiliates will be responsible for any loss or damage that could result from interception and interpretation by any third parties of any information being made available to you through this website. The Company and its affiliates cannot and do not guarantee the accuracy, timeliness or completeness of the information being made available to you in the Draft Red Herring Prospectus beyond the date of the Draft Red Herring Prospectus. The information contained in the Draft Red Herring Prospectus may not be updated since its original publication date and may not reflect the latest updates. The Company and its affiliates will not be responsible for any loss to any person or entity caused by any shortcoming, defect or inaccuracy which may have inadvertently or otherwise crept into the website. Neither the Company, any of its affiliates nor their directors, officers and employees will be liable or have any responsibility of any kind for any loss or damage that you incur in the event of any failure or disruption of this website, or resulting from the act or omission of any other party involved in making this website or the data contained therein available to you, or from any other cause relating to your access to, inability to access, or use of the website or these materials.
If you are not permitted to view the materials on this website or are in any doubt as to whether you are permitted to view these materials, please exit this webpage.
To access this information, you must confirm by pressing on the button marked “I Confirm” that, at the time of access you are located and resident in India. If you cannot make this confirmation, you must press the button marked “I Do Not Confirm”.
The documentation contained in these pages is posted solely to comply with Indian legal and regulatory requirements. Making the information contained herein available in electronic format does not constitute an offer to sell, the solicitation of an offer to buy, or a recommendation to buy or sell securities of the Company in the United States or in any other jurisdiction, including without limitation, India.
This will close in 0 seconds
PLEASE READ THIS NOTICE CAREFULLY. IT APPLIES TO ALL PERSONS WHO VIEW THIS WEBSITE. THESE MATERIALS ARE NOT DIRECTED AT OR INTENDED TO BE ACCESSED BY PERSONS LOCATED OUTSIDE INDIA. THESE MATERIALS ARE BEING MADE AVAILABLE ON THIS WEBSITE TO COMPLY WITH SECURITIES AND EXCHANGE BOARD OF INDIA (ISSUE OF CAPITAL AND DISCLOSURE REQUIREMENTS) REGULATIONS, 2018, AS AMENDED.
Investors should read the Draft Red Herring Prospectus and seek professional advice before taking any action.
IMPORTANT: You must read and agree with the terms and conditions of the following disclaimer before continuing.
The following disclaimer applies to the draft red herring prospectus of Molbio Diagnostics Limited (the “Company”) dated August 22, 2025 (the “Draft Red Herring Prospectus”) filed with the Securities and Exchange Board of India (“SEBI”) and hosted on this website in connection with the initial public offering of the equity shares (“Equity Shares”) of the Company (the “Offer”). You are advised to read the following notice carefully before accessing the Draft Red Herring Prospectus. In accessing the Draft Red Herring Prospectus, you agree to be bound by the following terms and conditions, including any modifications to them from time to time.
Access to the Draft Red Herring Prospectus does not constitute a recommendation by the Company, the members of the Syndicate (as defined in the Draft Red Herring Prospectus) or any of their respective affiliates or any other person to subscribe to the Equity Shares offered in the Offer.
The Draft Red Herring Prospectus is directed at, and is intended for distribution to, and use by, residents of India only. The information in this portion of our website, including the Draft Red Herring Prospectus, is not for publication or distribution, directly or indirectly, in or into the United States. The contents of the Draft Red Herring Prospectus is for your information only, and you acknowledge that access to the Draft Red Herring Prospectus is intended for use by you only and you agree not to forward the Draft Red Herring Prospectus on to any other person, internal or external to your company, in whole or in part, or otherwise provide access via e-mail or otherwise to any person. Any person into whose possession the DRHP comes is required to inform himself or herself about and to observe any such restrictions. BRLMs and/or its affiliates or their respective directors, officers and employees are not soliciting any action based on any of the information contained on this website, including the DRHP, and such information should not be construed as an offer, or invitation or offer to sell or the solicitation of any offer to buy or subscribe for or purchase any security. No part of the contents of the Draft Red Herring Prospectus shall be copied or duplicated in any form by any means or redistributed.
The Draft Red Herring Prospectus has been hosted on this website as prescribed under Regulation 26(1) of the Securities and Exchange Board of India (Issue of Capital and Disclosure Requirements) Regulations, 2018, as amended. Our Company has taken all necessary steps to ensure that the contents of the Draft Red Herring Prospectus as appearing on this website are identical to the Draft Red Herring Prospectus filed with the SEBI. You are reminded that documents transmitted in electronic form may be altered or changed during the process of transmission and consequently, neither the Company nor the BRLMs any of its affiliates accepts any liability or responsibility whatsoever in respect of alterations or changes which have taken place during the course of transmission of electronic data.
The Draft Red Herring Prospectus does not constitute an offer to sell or an invitation to subscribe to the securities offered in any jurisdiction to any person to whom it is unlawful to make an offer or invitation in such jurisdiction and is not intended for distribution to, or use by, any person or entity in any jurisdiction or country where (a) distribution or use of such information would be contrary to law or regulation; or (b) the Company or any of its affiliates would by virtue of such distribution become subject to new or additional registration, licensing or other regulatory requirements. The Equity Shares offered in the Offer have not been and will not be registered under the U.S. Securities Act of 1933, as amended (“U.S. Securities Act”) or any state securities laws in the United States, and unless so registered, may not be offered or sold within the United States, except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the U.S. Securities Act and in accordance with any applicable U.S. state securities laws. Accordingly, the Equity Shares are being offered and sold only outside the United States in ‘offshore transactions’ in compliance with Regulation S under the U.S. Securities Act and the applicable laws of the jurisdictions where such offers and sales are made.
Any person into whose possession the Draft Red Herring Prospectus comes is required to inform himself or herself about and to observe any such restrictions. Neither the Company, BRLMs nor any of its affiliates is soliciting any action based on the Draft Red Herring Prospectus, and it should not be construed as an offer to sell or the solicitation of an offer to buy any securities. Potential investors should not rely on the Draft Red Herring Prospectus for any investment decision.
Any decision on whether to invest in the equity shares described in the Draft Red Herring Prospectus may only be made after a red herring prospectus has been filed with the Registrar of Companies, Goa, Daman and Diu at Panaji and the SEBI and must be made solely on the basis of such red herring prospectus, as there may be material changes in the red herring prospectus compared to the Draft Red Herring Prospectus. Invitations to subscribe to or purchase the equity shares in the Offer will be made only pursuant to the red herring prospectus if the recipient is in India or the preliminary offering memorandum for the Offer, which comprises the red herring prospectus and the preliminary international wrap for the Offer, if the recipient is outside India. No person outside India is eligible to Bid for equity shares in the Offer unless that person has received the preliminary offering memorandum for the Offer, which shall contain the selling restrictions for the Offer outside India.
Any potential investor should note that investment in equity shares involves a high degree of risk and for details relating to such risk, please see the section titled “Risk Factors” of the red herring prospectus, when available.
Neither the Company, BRLMs, nor any of its affiliates will be responsible for any loss or damage that could result from interception and interpretation by any third parties of any information being made available to you through this website. The Company and its affiliates cannot and do not guarantee the accuracy, timeliness or completeness of the information being made available to you in the Draft Red Herring Prospectus beyond the date of the Draft Red Herring Prospectus. The information contained in the Draft Red Herring Prospectus may not be updated since its original publication date and may not reflect the latest updates. The Company and its affiliates will not be responsible for any loss to any person or entity caused by any shortcoming, defect or inaccuracy which may have inadvertently or otherwise crept into the website. Neither the Company, any of its affiliates nor their directors, officers and employees will be liable or have any responsibility of any kind for any loss or damage that you incur in the event of any failure or disruption of this website, or resulting from the act or omission of any other party involved in making this website or the data contained therein available to you, or from any other cause relating to your access to, inability to access, or use of the website or these materials.
If you are not permitted to view the materials on this website or are in any doubt as to whether you are permitted to view these materials, please exit this webpage.
To access this information, you must confirm by pressing on the button marked “I Confirm” that, at the time of access you are located and resident in India. If you cannot make this confirmation, you must press the button marked “I Do Not Confirm”.
The documentation contained in these pages is posted solely to comply with Indian legal and regulatory requirements. Making the information contained herein available in electronic format does not constitute an offer to sell, the solicitation of an offer to buy, or a recommendation to buy or sell securities of the Company in the United States or in any other jurisdiction, including without limitation, India.
This will close in 0 seconds
PLEASE READ THIS NOTICE CAREFULLY. IT APPLIES TO ALL PERSONS WHO VIEW THIS WEBSITE. THESE MATERIALS ARE NOT DIRECTED AT OR INTENDED TO BE ACCESSED BY PERSONS LOCATED OUTSIDE INDIA. THESE MATERIALS ARE BEING MADE AVAILABLE ON THIS WEBSITE TO COMPLY WITH SECURITIES AND EXCHANGE BOARD OF INDIA (ISSUE OF CAPITAL AND DISCLOSURE REQUIREMENTS) REGULATIONS, 2018, AS AMENDED.
Investors should read the Draft Red Herring Prospectus and seek professional advice before taking any action.
IMPORTANT: You must read and agree with the terms and conditions of the following disclaimer before continuing.
The following disclaimer applies to the draft red herring prospectus of Molbio Diagnostics Limited (the “Company”) dated August 22, 2025 (the “Draft Red Herring Prospectus”) filed with the Securities and Exchange Board of India (“SEBI”) and hosted on this website in connection with the initial public offering of the equity shares (“Equity Shares”) of the Company (the “Offer”). You are advised to read the following notice carefully before accessing the Draft Red Herring Prospectus. In accessing the Draft Red Herring Prospectus, you agree to be bound by the following terms and conditions, including any modifications to them from time to time.
Access to the Draft Red Herring Prospectus does not constitute a recommendation by the Company, the members of the Syndicate (as defined in the Draft Red Herring Prospectus) or any of their respective affiliates or any other person to subscribe to the Equity Shares offered in the Offer.
The Draft Red Herring Prospectus is directed at, and is intended for distribution to, and use by, residents of India only. The information in this portion of our website, including the Draft Red Herring Prospectus, is not for publication or distribution, directly or indirectly, in or into the United States. The contents of the Draft Red Herring Prospectus is for your information only, and you acknowledge that access to the Draft Red Herring Prospectus is intended for use by you only and you agree not to forward the Draft Red Herring Prospectus on to any other person, internal or external to your company, in whole or in part, or otherwise provide access via e-mail or otherwise to any person. Any person into whose possession the DRHP comes is required to inform himself or herself about and to observe any such restrictions. BRLMs and/or its affiliates or their respective directors, officers and employees are not soliciting any action based on any of the information contained on this website, including the DRHP, and such information should not be construed as an offer, or invitation or offer to sell or the solicitation of any offer to buy or subscribe for or purchase any security. No part of the contents of the Draft Red Herring Prospectus shall be copied or duplicated in any form by any means or redistributed.
The Draft Red Herring Prospectus has been hosted on this website as prescribed under Regulation 26(1) of the Securities and Exchange Board of India (Issue of Capital and Disclosure Requirements) Regulations, 2018, as amended. Our Company has taken all necessary steps to ensure that the contents of the Draft Red Herring Prospectus as appearing on this website are identical to the Draft Red Herring Prospectus filed with the SEBI. You are reminded that documents transmitted in electronic form may be altered or changed during the process of transmission and consequently, neither the Company nor the BRLMs any of its affiliates accepts any liability or responsibility whatsoever in respect of alterations or changes which have taken place during the course of transmission of electronic data.
The Draft Red Herring Prospectus does not constitute an offer to sell or an invitation to subscribe to the securities offered in any jurisdiction to any person to whom it is unlawful to make an offer or invitation in such jurisdiction and is not intended for distribution to, or use by, any person or entity in any jurisdiction or country where (a) distribution or use of such information would be contrary to law or regulation; or (b) the Company or any of its affiliates would by virtue of such distribution become subject to new or additional registration, licensing or other regulatory requirements. The Equity Shares offered in the Offer have not been and will not be registered under the U.S. Securities Act of 1933, as amended (“U.S. Securities Act”) or any state securities laws in the United States, and unless so registered, may not be offered or sold within the United States, except pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the U.S. Securities Act and in accordance with any applicable U.S. state securities laws. Accordingly, the Equity Shares are being offered and sold only outside the United States in ‘offshore transactions’ in compliance with Regulation S under the U.S. Securities Act and the applicable laws of the jurisdictions where such offers and sales are made.
Any person into whose possession the Draft Red Herring Prospectus comes is required to inform himself or herself about and to observe any such restrictions. Neither the Company, BRLMs nor any of its affiliates is soliciting any action based on the Draft Red Herring Prospectus, and it should not be construed as an offer to sell or the solicitation of an offer to buy any securities. Potential investors should not rely on the Draft Red Herring Prospectus for any investment decision.
Any decision on whether to invest in the equity shares described in the Draft Red Herring Prospectus may only be made after a red herring prospectus has been filed with the Registrar of Companies, Goa, Daman and Diu at Panaji and the SEBI and must be made solely on the basis of such red herring prospectus, as there may be material changes in the red herring prospectus compared to the Draft Red Herring Prospectus. Invitations to subscribe to or purchase the equity shares in the Offer will be made only pursuant to the red herring prospectus if the recipient is in India or the preliminary offering memorandum for the Offer, which comprises the red herring prospectus and the preliminary international wrap for the Offer, if the recipient is outside India. No person outside India is eligible to Bid for equity shares in the Offer unless that person has received the preliminary offering memorandum for the Offer, which shall contain the selling restrictions for the Offer outside India.
Any potential investor should note that investment in equity shares involves a high degree of risk and for details relating to such risk, please see the section titled “Risk Factors” of the red herring prospectus, when available.
Neither the Company, BRLMs, nor any of its affiliates will be responsible for any loss or damage that could result from interception and interpretation by any third parties of any information being made available to you through this website. The Company and its affiliates cannot and do not guarantee the accuracy, timeliness or completeness of the information being made available to you in the Draft Red Herring Prospectus beyond the date of the Draft Red Herring Prospectus. The information contained in the Draft Red Herring Prospectus may not be updated since its original publication date and may not reflect the latest updates. The Company and its affiliates will not be responsible for any loss to any person or entity caused by any shortcoming, defect or inaccuracy which may have inadvertently or otherwise crept into the website. Neither the Company, any of its affiliates nor their directors, officers and employees will be liable or have any responsibility of any kind for any loss or damage that you incur in the event of any failure or disruption of this website, or resulting from the act or omission of any other party involved in making this website or the data contained therein available to you, or from any other cause relating to your access to, inability to access, or use of the website or these materials.
If you are not permitted to view the materials on this website or are in any doubt as to whether you are permitted to view these materials, please exit this webpage.
To access this information, you must confirm by pressing on the button marked “I Confirm” that, at the time of access you are located and resident in India. If you cannot make this confirmation, you must press the button marked “I Do Not Confirm”.
The documentation contained in these pages is posted solely to comply with Indian legal and regulatory requirements. Making the information contained herein available in electronic format does not constitute an offer to sell, the solicitation of an offer to buy, or a recommendation to buy or sell securities of the Company in the United States or in any other jurisdiction, including without limitation, India.
This will close in 0 seconds